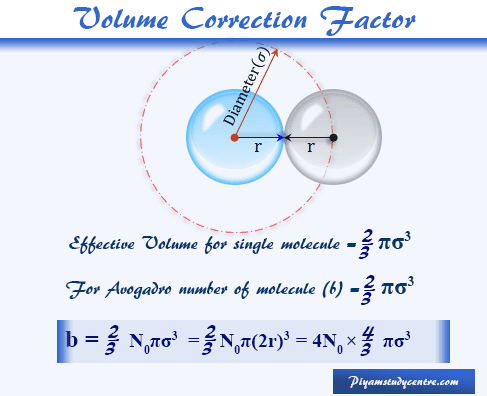
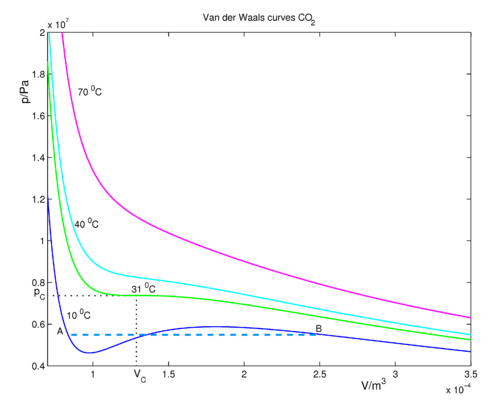
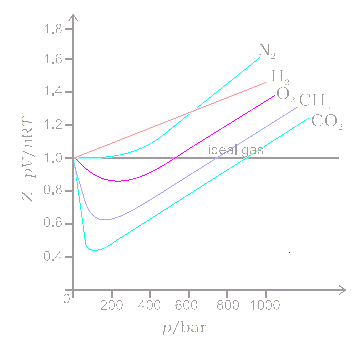
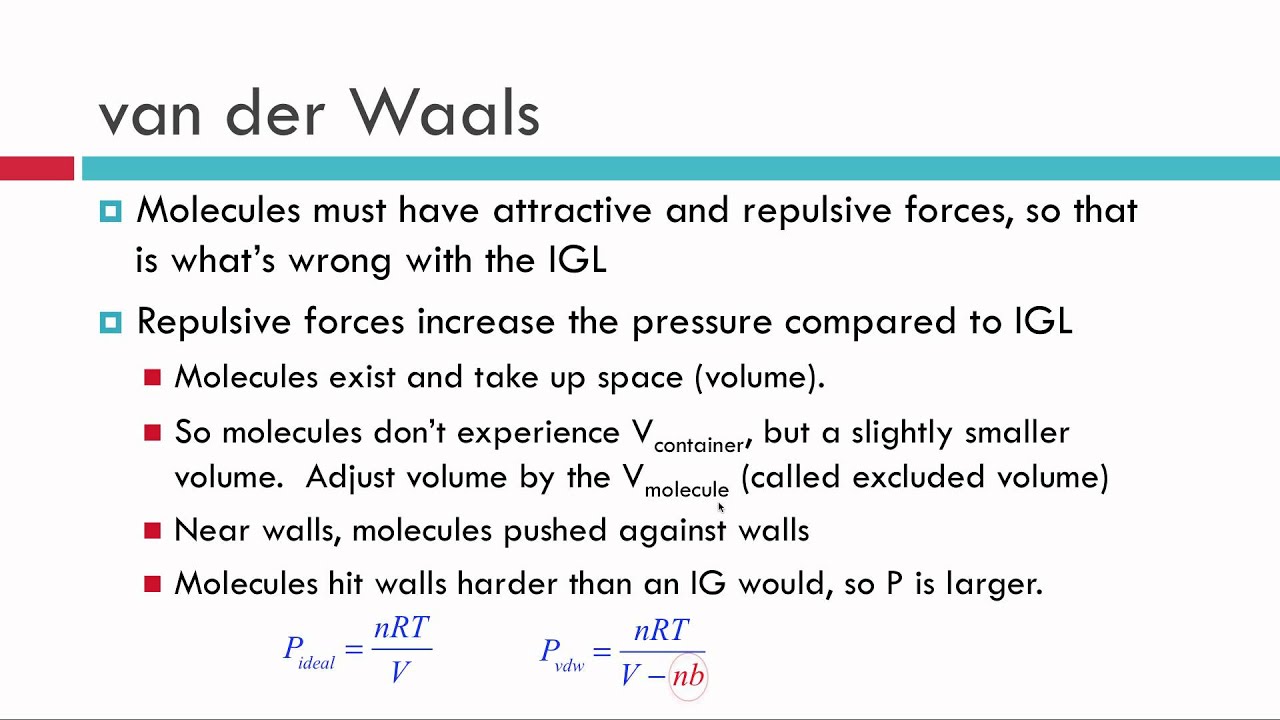
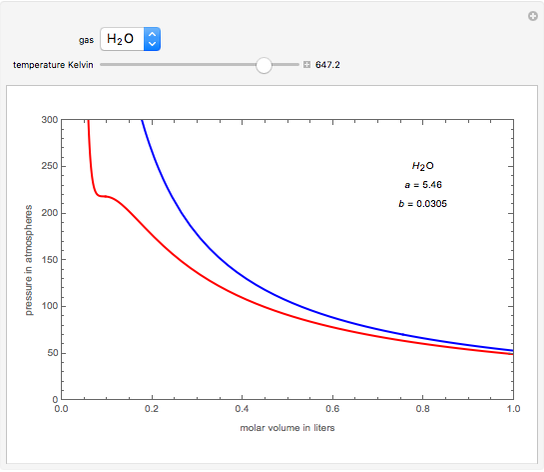
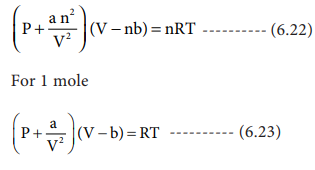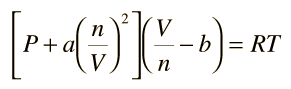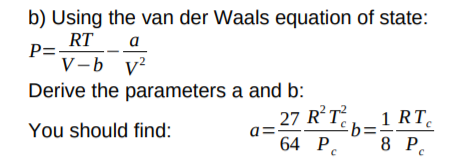The van der Waals equation of state: Chemical potential µ as a function... | Download Scientific Diagram
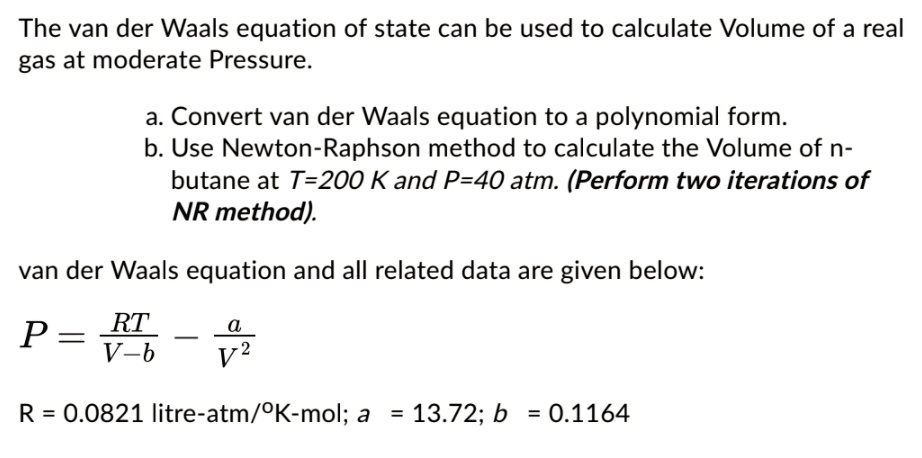
SOLVED: The van der Waals equation of state can be used to calculate Volume of a real gas at moderate Pressure: a. Convert van der Waals equation to polynomial form b. Use

van der Waals Equation of State Revisited: Importance of the Dispersion Correction | The Journal of Physical Chemistry B
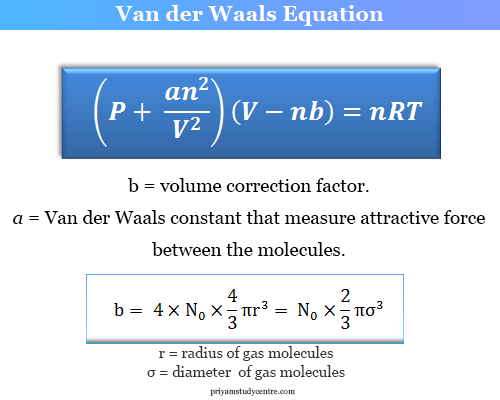
 = RT , at high pressure, the Van der Waals equation gets reduced to : Using Van der Waals equation, [ P + a/V^2 ](V - b) = RT , at high pressure, the Van der Waals equation gets reduced to :](https://dwes9vv9u0550.cloudfront.net/images/9876945/d6a3d03b-a449-4d51-986d-b05943344e70.jpg)